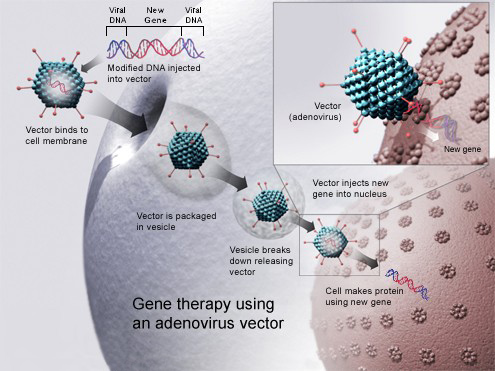
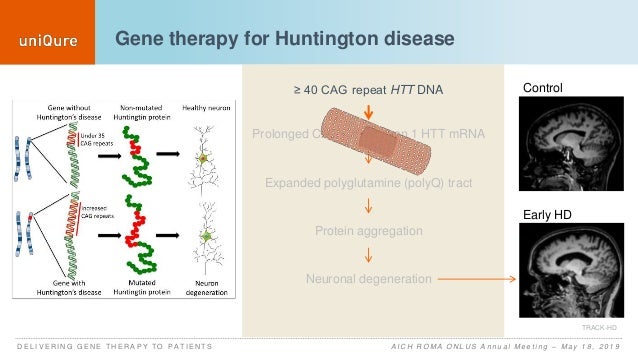
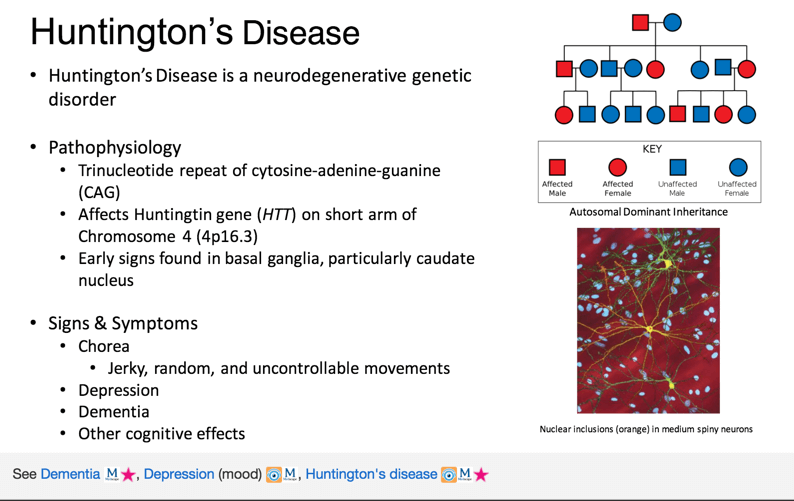
Gene therapy huntington's disease. Researchers have taken an important first step toward protecting against Huntingtons disease using gene therapy. The ideal method of administration of gene therapy has been hotly debated and viral vectors have provided one method of long-term. AMT-130 targets the accumulation of the exon 1.
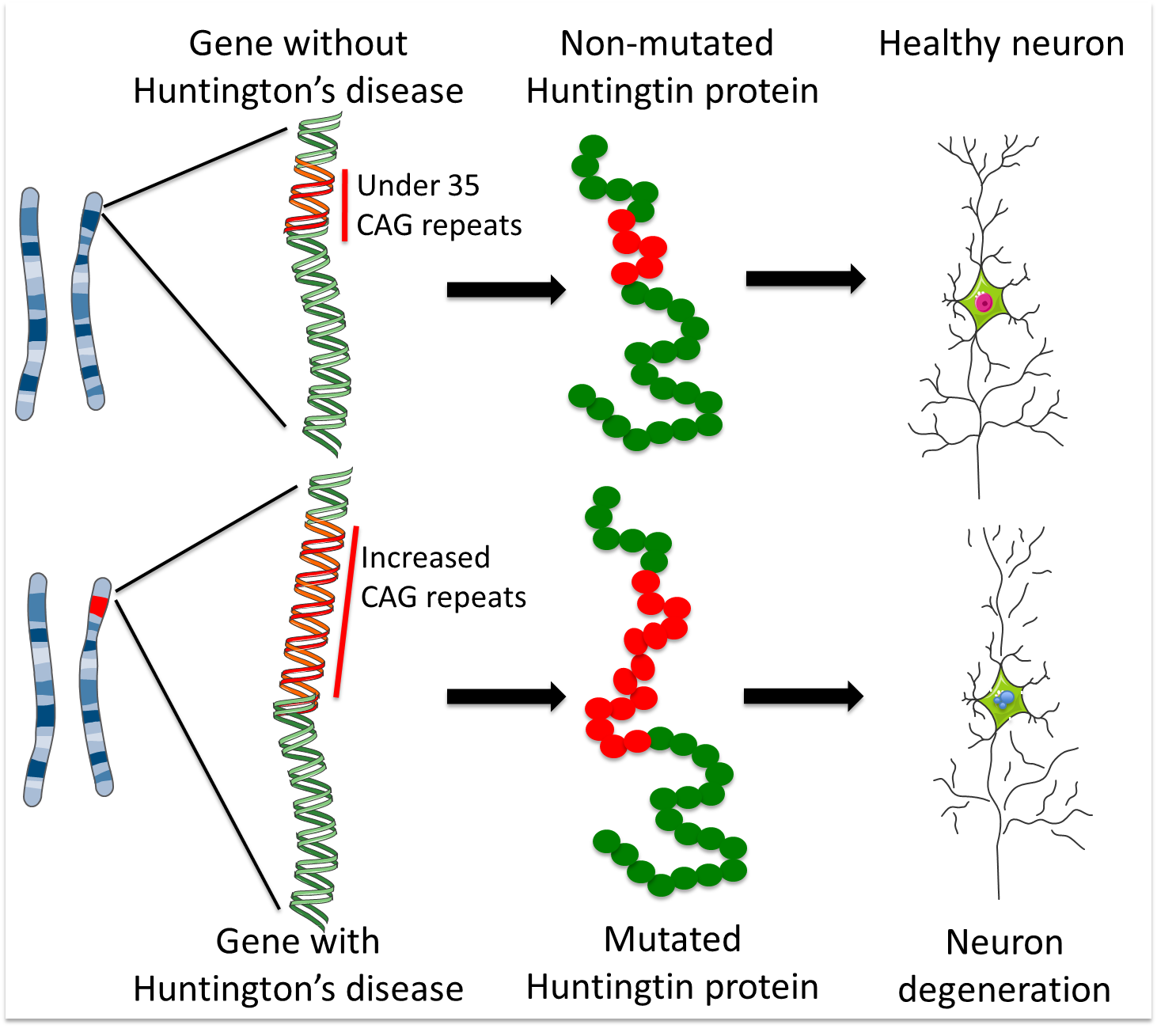
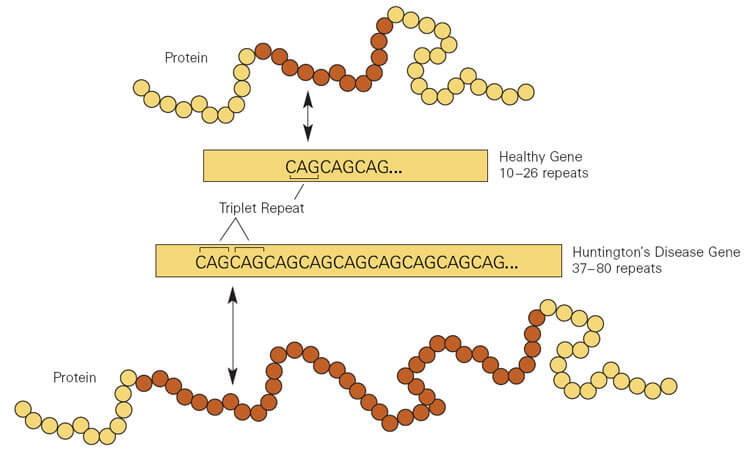
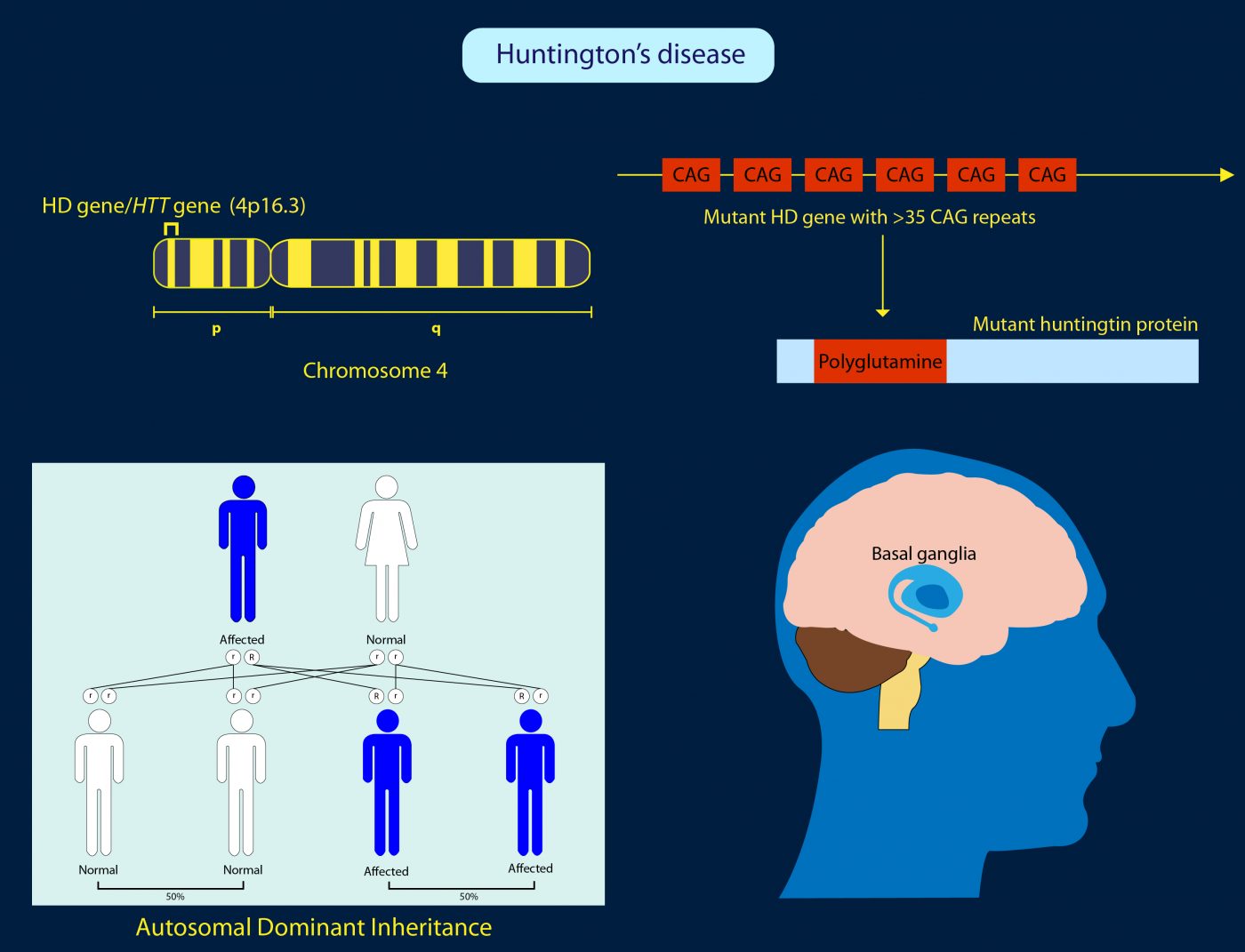
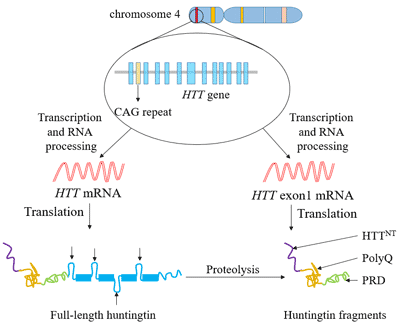
Huntingtons disease HD is a rare disease characterized by abnormal chorea movement and caused by Huntingtin Htt gene mutation and neurodegeneration in a brain area called striatum. AMT-130 targets the deep brain structures known for the disease pathology onset. CAG Expansion in the Huntington Disease Gene Is Associated with a Specific and Targetable Predisposing Haplogroup Simon C.
A leader in gene therapy Ohio State also has multiple studies for Parkinsons Huntingtons and aromatic L-amino acid decarboxylase deficiency a rare genetic disorder affecting children and resulting in developmental delay weak muscle tone and difficulty moving Mohler said. Butland Henk Visscher Jennifer A. Collins Alicia Semaka Thomas J.
Huntingtons Disease is an. Twenty-five years ago as molecular biologists first honed the tools that now allow them to manipulate DNA at will it was claimed that gene therapy could soon free humanity from the misery of. The clinical hold will be kept in place until certain issues with VY-HTT01s chemistry manufacturing and controls are resolved Voyager said in a press release.
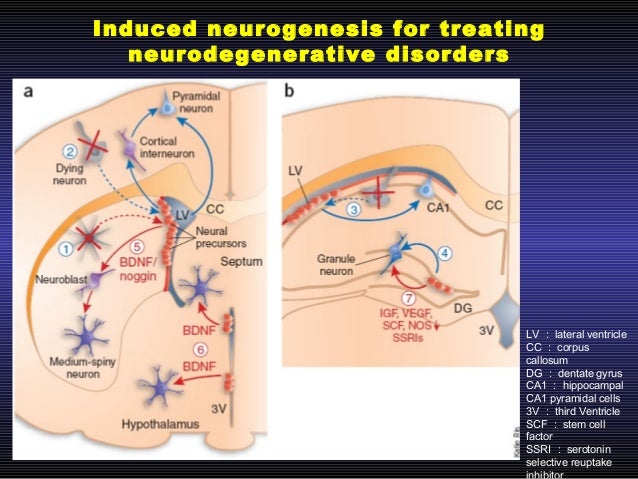
Food and Drug Administration FDA is putting on hold Voyager Therapeutics request to test VY-HTT01 its investigational gene therapy for Huntingtons disease in a clinical trial. Researchers have developed a new regenerative gene therapy using neurogenic differentiation which has shown efficacy treating Huntingtons disease in mice. A a newly converted neuron red surrounded by astrocytes green.
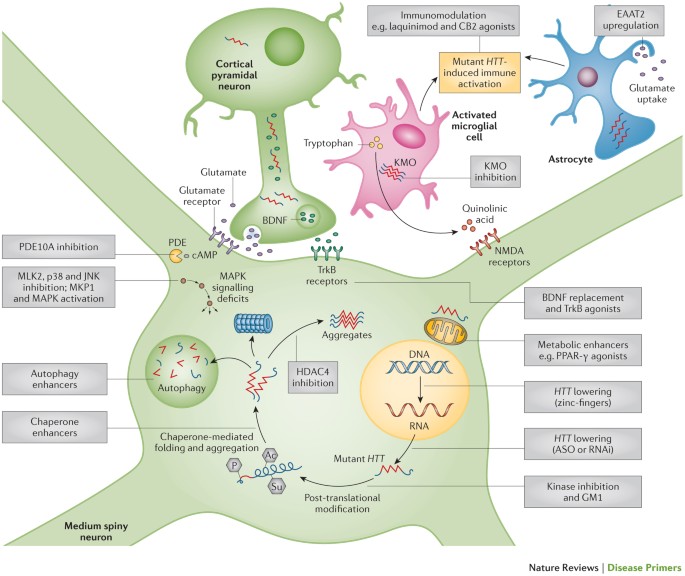
AMT-130 is a gene therapy designed to lower the production of the mutated form of the huntingtin protein the underlying cause of Huntingtons disease. One possible mechanism is a diminished nuclear translocation of the transcription factor sterol regulatory element binding protein 2 SREBP2 and consequently reduced activation of SREBP-controlled genes in the cholesterol biosynthesis pathway. We are leveraging our modular and validated technology platform to rapidly advance a pipeline of proprietary gene therapies to treat patients with hemophilia B Huntingtons disease Fabry disease.
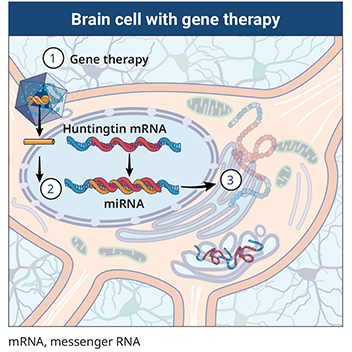
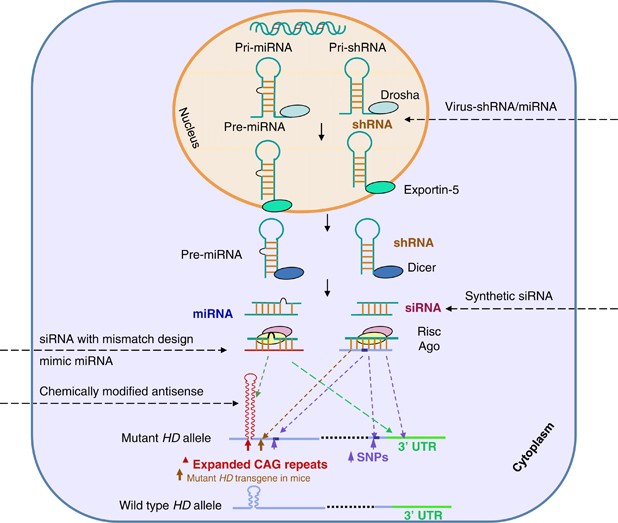
An artificial miRNA targeting the huntingtin. The method which exploits a.
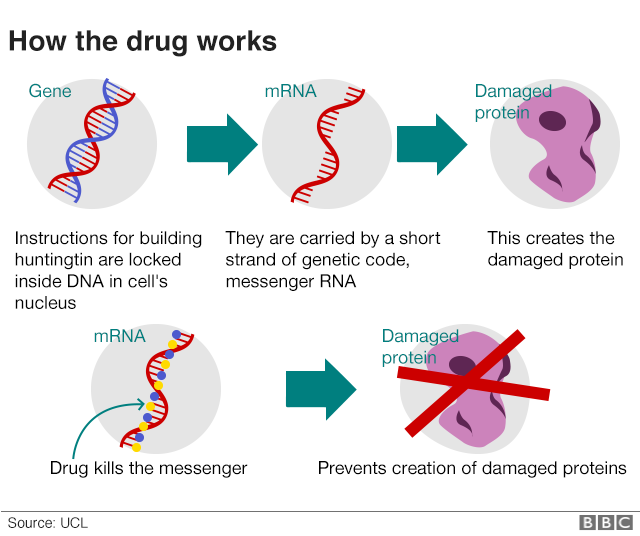
Using gene therapy to switch off genes instead of adding new ones could slow down or prevent the fatal brain disorder Huntingtons disease.
AMT-130 targets the deep brain structures known for the disease pathology onset. We are leveraging our modular and validated technology platform to rapidly advance a pipeline of proprietary gene therapies to treat patients with hemophilia B Huntingtons disease Fabry disease. Huntingtons Disease is an. AMT-130 targets the deep brain structures known for the disease pathology onset. Aronin has partnered with Dr Mueller an expert on vector-derived RNAi using AAV vectors to develop such a therapy for Huntingtons disease. The therapy is made of a small portion of synthetic genetic material called microRNA miRNA that is inserted into cells and carried using an adeno-associated virus which has been modified to be harmless. Warby Alexandre Montpetit Anna R. One potential powerful approach is gene therapy. The method which exploits a.
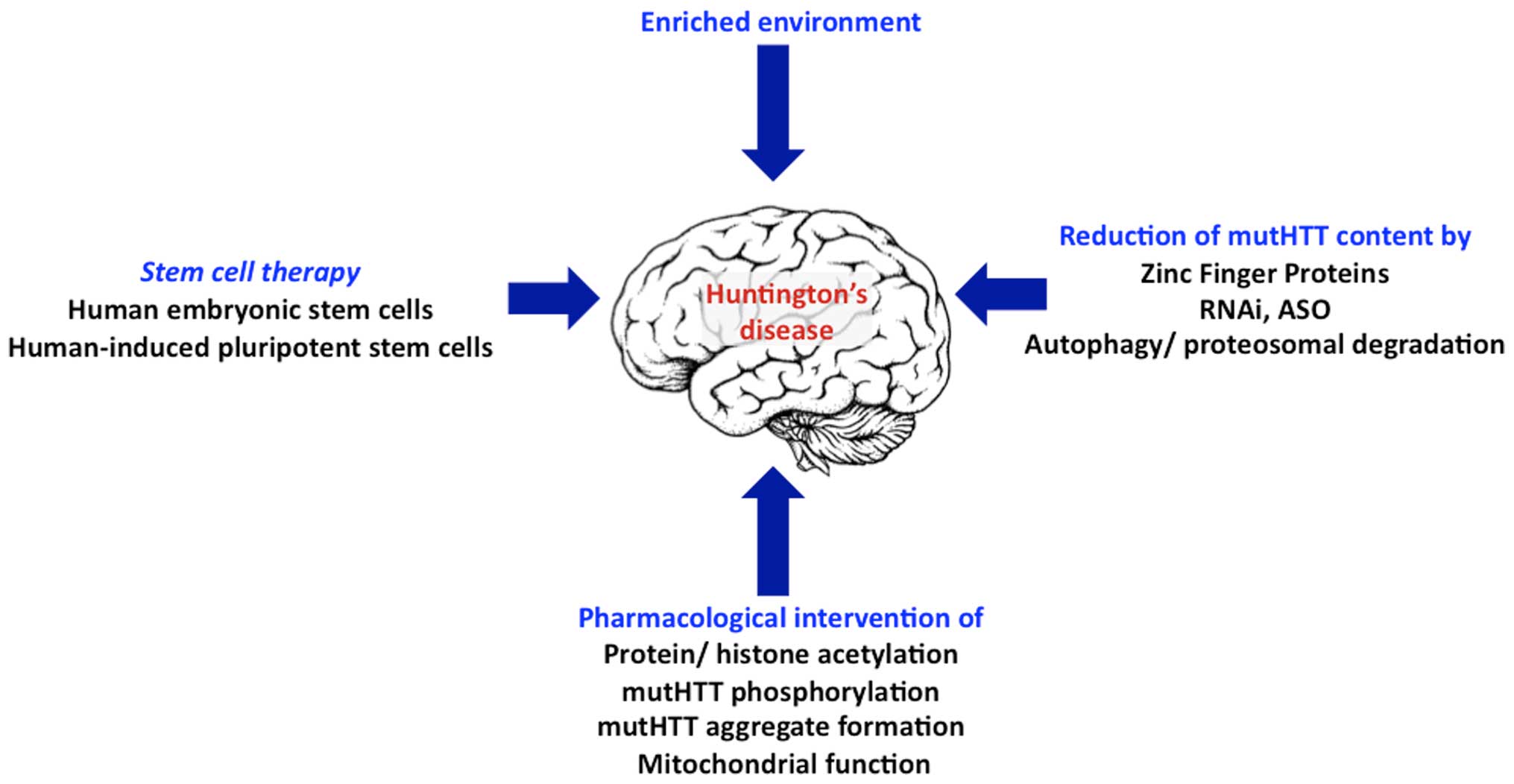
Researchers have taken an important first step toward protecting against Huntingtons disease using gene therapy. Huntingtons Disease is an. Huntingtons disease HD is a neurodegenerative disease for which there is no cure. A new gene therapy to potentially treat Huntingtons disease has regenerated the. Huntingtons disease HD is a rare disease characterized by abnormal chorea movement and caused by Huntingtin Htt gene mutation and neurodegeneration in a brain area called striatum. Therapies that are efficacious in animal models have to date shown benefit for humans. A gene repressor strategy using viral vectors has been developed for Huntingtons disease HD using engineered zinc finger fusion proteins specific for the CAG repeat of.

























0 Yorumlar